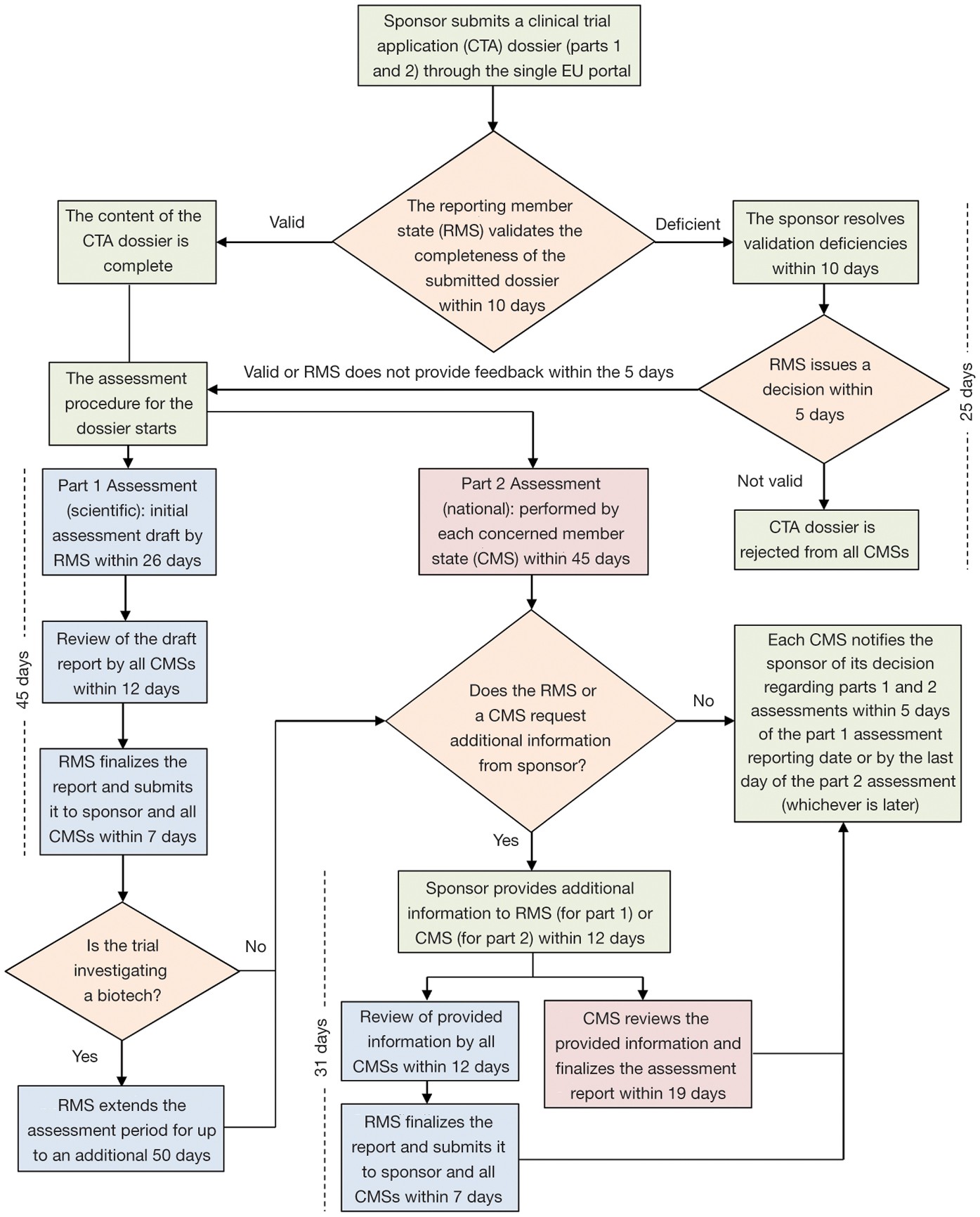
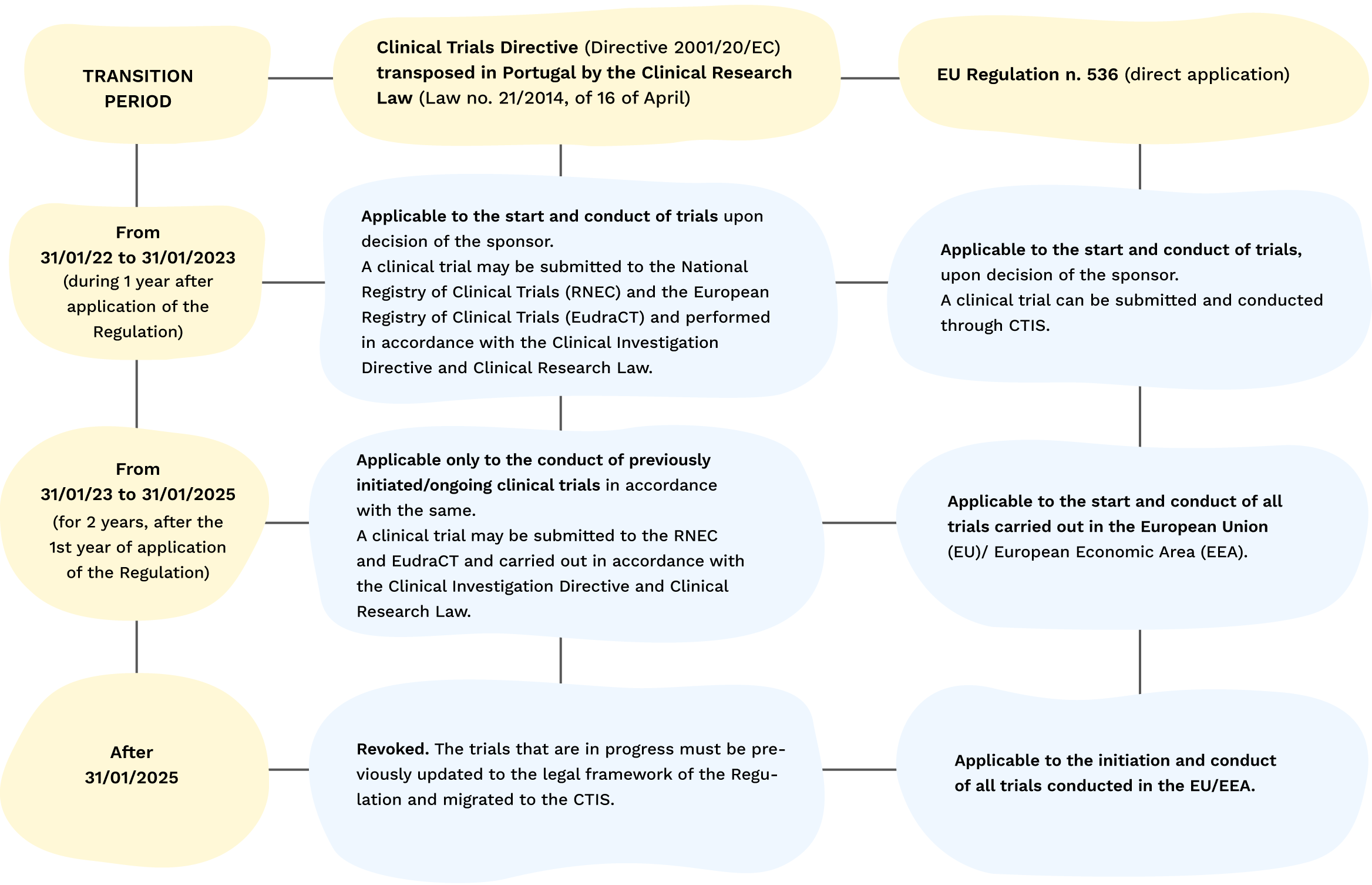
EORTC EU Clinical Trials Directives Organisation and Implementation of Cancer Clinical Trials Anastassia Negrouk EORTC Regulatory Affairs Manager Intergroup. - ppt download

Retrovirus mediated hematopoietic gene therapy: A European regulatory perspective with special focus on the situation in Germany
The EU Clinical Trials Regulation: Implications of the New Transparency Rules on Patenting - Lexology

Timeline impact assessment and Revision of Directive 2001/20/EC (see... | Download Scientific Diagram

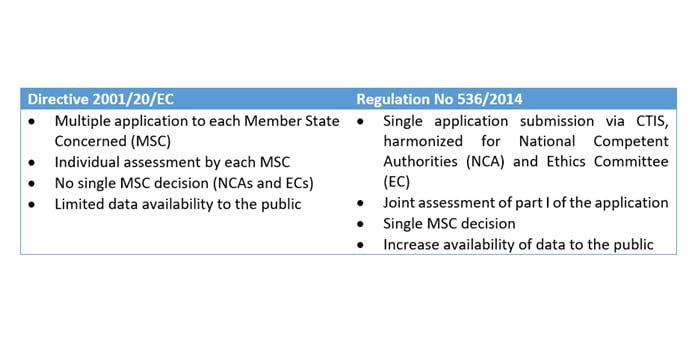
GCP and Quality in “Regulation (EU) 536/2014 on clinical trials on medicinal products for human use and repealing Directive 2001/20/EU” - ScienceDirect